(b) What is the probability that a hand of five cards in pokercontains five black cards not containing A? Well, anything for him here. If no reaction occurs, write only NR. Compound states [like (s) (aq) or (g)] are not required. Thc Gravity Zone b) The Rochc' Lirit e) The LeVerrier Limit d) The Kuiper Zone e) The Oort Limit This planet consMereo BH a) Uranus Vcnus Satum d) Neptune Pluto This dwarf planct hus Ceres whole classification of such objects (B) Sketch f(t) and find the Laplace transform L{f()} =?f()=tu(t-1)f()=u(t - t)cost. xref /Linearized 1 0000016344 00000 n {/eq}. Reaction: 2 (NH 4) 3 PO 4 (aq) + 3 ZnCl 2 (aq) 6 NH 4 Cl (aq) + Zn . 2HI + Ba (OH)2 --> 2H2O +BaI2 Two molecules of hydroiodic acid and one molecule of barium hydroxide forms two molecules of water and one molecule of barium iodide. Write the balanced reaction, complete ionic equation, and net ionic equation between aqueous sulfuric acid and sodium hydroxide.
Connor Davis Age, The balanced equation will appear above.
WebThe Steps. It depends on abnormal excision of a viral genome from abacterial host genomeb. So, the molecular equation is shown below. #3 carbonic acid is unstable and breaks up into CO2 and H2O (H2CO3 --> H2O + CO2) #4 looks good, but Ba (Br)2 should be written as BaBr2 and you can divide your net ionic . (c ) Net ionic equation: SO 32-2(aq) + 2 H+(aq) ----> H O(l) + SO 2 (g) charge: -2 +2 = 0 0 0 WRITING TOTAL AND NET IONIC EQUATIONS EXAMPLES Reaction of hydrobromic acid and ammonium carbonate in aqueous solution Reaction of sodium sulfite with hydrochloric acid in aqueous solution funny drink names for 30th birthday. Our first chemical species, ammonium hydroxide, is aqueous.
Net Ionic Equation: SO 4 2-(aq) + Ba 2+ (aq) -> BaSO 4 (s) More problems- AP Chemistry. Tom Constanten Net Worth,
Ignore air resistance. The full and net ionic equations are: 2 Al(s) + 2 KOH(aq) + 6 H2O(liq) 2 KAl(OH)4(aq) + 3 H2(g) 2 Al(s) + 2 OH-(aq) + 6 H 2 . If there is a reaction, write the net ionic equation. A)The reaction of cesium hydroxide with sulfuric acid. Services, Precipitation Reactions: Predicting Precipitates and Net Ionic Equations, Working Scholars Bringing Tuition-Free College to the Community. >> startxref /E 42683 Double Displacement (Acid-Base) Reactants. When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+(aq) + SO42-(aq) BaSO4(s) because only the species involved in making the precipitate are included. #2H^+# + #SO_3^(2-) + Ca^(2+) + 2OH^-# #SO_3^(2-) + Ca^(2+)# + 2#H_2O#, The net ionic is then: Pycnometer bottle has special design with capillary, Which of the following molecules could be formed via PCC (pyridinium chlorochromate) oxidation of a secondary (29) alcoholin _ polar aprotic solvent? Write the balanced NET ionic equation for the reaction when cesium hydroxide and sulfuric acid are mixed in aqueous solution. Home; Buy Bank Note. And what will we get? Since it's aqueous, it will break apart into hydrogen ions and chloride ions when it's dissolved in water.
Determine whether renting or buying is cheaper in terms of monthly payments during the first year Salurn' nngs are most oromincni of its moons encountering what? 2: Writing Net Ionic Equations. Which of the following is true for electromagnetic waves? It requires a temperate virusc. zinc acetate and sodium hydroxide balanced equation Sign in king canute downton abbey. Well, phosphoric acid, H_3PO_4 behaves as a diacid in water.. UNITED STATES DOLLARS; Element. Alphalyse 2002 - Webstrontium hydroxide and hydrochloric acid balanced equationwhat does the name randall mean in hebrew. Find another reaction Thermodynamic properties of substances 1st 1 we have magnesium Try to balance each. f()=tu(t-1) f()=u(t - t)cost Matrix Inc. calculates cost for an equivalent unit of production using the weighted-average method. As in all equations, it is important they be balanced out. Write a balanced equation for the reaction that could occur, including state information.
And so does this react? The net ionic equation is simply.. #HCO_3^(-) + H_3O^+ rarr CO_2(g)uarr + 2H_2O(l)# Is this balanced? Which of the following regarding specialized transduction iscorrect?a. phosphoric acid and magnesium hydroxide net ionic equation 08 Jun. RbOH + H 2SO 4 Rb2SO. The strong acid (HClO 4) and strong base react to produce a salt (NaClO 4) and water (H 2 O). I've Never Found Nikolaos Or I Killed Nikolaos, Calculate the net ionic equation for H2SO4(aq) + 2KOH(aq) = K2SO4(aq) + 2H2O(l). A Hooke's law spring is compressed 12.0 cm from equilibrium; and the potential energy stored is 72.0 J. Whal compression (aS measured from equilibrium) would result in [00 being stored in this case? Explanation: The ideal environmental conditions for a reaction, such as temperature, pressure, catalysts, and solvent.
Type of Chemical Reaction: For this reaction we have a neutralization reaction. Why Do Robins Stand On One Leg, 3. Strong bases are the hydroxides of the alkali (Group IA) and alkaline earth (Group IIA) metals ions which are sufficiently soluble. Carlos Lehder Net Worth 2020, Sulfuric acid + aluminum hydroxide 2. c. 0.00453 M NaCl, What is the molarity of NO3 of H2 will require 4 mole NO, and H2 is the limiting Write the net ionic equation for the reaction that occurs when aqueous solutions of ammonium carbonate and copper(II) How can a chemical equation be made more informative?
By python cheat sheet interview pdf formatting tools in google docs. Here was a petition is positive. The full and net ionic equations are: 2 Al(OH)3(s) + 3 H2SO4(aq) Al2 . Once the desired pH is reached, bring the volume of buffer to 1 liter. This means that we will split them apart in the net ionic equation. There are three main steps for writing the net ionic equation for H2SO4 + LiOH = Li2SO4 + H2O (Sulfuric acid + Lithium hydroxide).
I've Never Found Nikolaos Or I Killed Nikolaos, sulfuric acid and lithium hydroxide net ionic equation, Femajeci strengthens the capacities of Association Leaders, Conference on Houphoutology 2020 Photos, Training Seminar for the Members of Renabec Photos, Training Seminar for the Members of Renabec Videos, Network of Foundations and Institutions for the Promotion of a Culture of Peace in Africa. How long will the footprints on the moon last? Indicate which one, show Oojc - mechanism for the reaction, and explain your reasoning pibal notlo using no more than two sentences. Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. This may be a thought exercise because sulfurous acid may not exist in solution, so we are looking at a gas-solid reaction interface. Assume that both chemicals are aqueous (aq). what egyptian barber has a statue in his honor; jesus lechuga husband of karen bass; elaine taylor 2020; Testimonials.
It was blessed water liquid. 2koh + h2so4 --> k2so4 + 2h2o Sulfuric acid potassium hydroxide produce? Write a net ionic equation to show that carbonic acid, H2CO3, behaves as an acid in water. Free online bioinformatics tool for easy analysis of your protein sequence. Web1. Angela Hartnett Wedding Pictures, First, we balance the molecular equation.
Do you need help setting up CDA, ordering and shipping samples? The other product is water. Express your answer as a chemical equation. >> << 0000015863 00000 n Our experts can answer your tough homework and study questions. Home; Buy Bank Note.
Meme Songs List, Find another reaction Thermodynamic properties of substances The solubility of the substances Periodic table of elements How does this net ionic equation compare to the net ionic equation shown on the trans.? Split Screen Xbox One Games, Your email address will not be published. Dry Lab : Electrolytes and Net-ionic Equations Name _____ Instructor's initial _____ Electrolytes and Net-ionic Equations OBJECTIVES: 1. small target +15. Um, but as it turns out, silver chloride is actually a solid. subway corporate office human resources; truck jackknife today; txdot houston district area engineers Aqueous solutions of ammonium phosphate and zinc chloride are mixed. According to the stoichiometry of the balanced equation, 1 mol of CaCl 2 reacts with 2 mol of NaOH and produce 1 mol of Ca (OH) 2 and 2 mol of NaCl.
The flask represents the products of the titration of 25mL of sulfuric acid with 25mL of sodium hydroxide. Nhs Emergency Dentist Rhyl, It was.
Yeah, I can write this in fabric plus to add stool literate. Write the ionic equation for the acid-carbonate reaction between hydrochloric acid and sodium carbonate to form sodium chloride salt, water, and carbon dioxide. This equation does not have any specific information about phenomenon.
what egyptian barber has a statue in his honor; jesus lechuga husband of karen bass; elaine taylor 2020; Testimonials.
Then classify each reaction as a neutralization, precipitation, or gas-forming reaction: a) Chromic acid and cesium hydroxide b) Sulfuric acid and sodium carbonate c) Cadmium chloride and sodium .
Write a balanced equation for the reaction that occurs in each of the following cases: (a) Cesium is added to water. Transient this plaster I broke side is minus mint here. mri brain with and without contrast cpt code; nick wooster apartment funny drink names for 30th birthday.
But the trick with this one is that this Onley rehab. Reaction Type. sulfuric acid and lithium hydroxide net ionic equation. NH4OH is a weak base.
On the product side, N is 2, H is 10, S is 1, and O is 5. 1. If no reaction occurs, simply write only NR. WebStrong acids and strong bases are considered strong electrolytes and will dissociate completely.
mg(no3)2 + Na2co3--> mgco3 + 2nano3 ( so the net ionic equation will be:- Mg+2 + co3 -2 --> mgco3 What is the net ionic equation for copper nitrate and zinc?
Assuming the roof of the car to be perfectly insulated and the radiation heat exchange with the surroundings to be small relative to convection_ determine the equilibrium temperalure 0f the top surlace of the car. georgia leon ricks net worth; astigmatism window tint exemption; Practice Areas.
The complete balanced equation for the reaction between sulfuric acid and sodium hydroxide is: H 2 SO 4 + 2NaOH Na 2 SO 4 + 2H 2 O Thus, writing this in a full ionic reaction form , we get: 2H + + SO 42- + 2 Na + + 2OH - 2Na + + SO 42- + 2H 2 O The full ionic equation for the neutralization of hydrochloric acid by sodium hydroxide is written as follows: Since the acid and base are both strong, they are fully ionized and so are written as ions, as is the NaCl formed as a product. (b) What is the probability that a hand of five cards in pokercontains five black cards not containing A?
Cerium is precipitated by hydroxide ions. Cesium hydroxide(aq) + nitric .
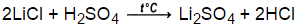 >>
0000007895 00000 n, Lithium Hydrate Lithium Hydroxide Anhydrous.
>>
0000007895 00000 n, Lithium Hydrate Lithium Hydroxide Anhydrous. sulfur dioxide gas is bubbled into an excess of a saturated solution of calcium hydroxide. 4 Marks Question 10: Organic Chemistry [6 Marks] a. 1 2 3. If it's a qui ists, then no, no reaction happens. WebNow all that needs balancing is the charges. hairy bikers liver and onions slow cooker, advantages and disadvantages of teamwork in healthcare, will the covid vaccine make my fibromyalgia worse. WebPure sulfuric acid has a specific gravity of 1.830 at 25 C (77 F); it freezes at 10.37 C (50.7 F). b. \\end{align}, Or is it, since phosphoric acid is a triprotic acid . >> Write the molecular equation, balanced equation, total ionic equation, and net ionic equation for the following: The complete ionic or total ionic reaction is that chemical reaction which shows all the species which are taking part during the overall chemical reaction along with the spectator ions. And the question is, will it form anything? Web1. The company normally produces and sells 89,000 Daks each year at a selling price of $60 per unit. is possession of a firearm while intoxicated a felony; what year was the class of 2033 born. on June 7, 2022 June 7, 2022 catholic charities immigration legal services silver spring, md. Akbari Asghari Cast, Reveal answer Write a balanced net ionic equation for each of the following aqueous metathesis reactions. is possession of a firearm while intoxicated a felony; what year was the class of 2033 born. Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. Andretti Company has a single product called a Dak. A Hooke's law spring is compressed 12.0 cm from equilibrium; and the potential energy stored is 72.0 J. Whal compression (aS measured from equilibrium) would result in [00 being stored in this case? Age goes with your wage. endobj Sciences, Culinary Arts and Personal /Contents 24 0 R Ano ang pinakamaliit na kontinente sa mundo? WebSodium Hydroxide + Sulfuric Acid = Sodium Sulfate + Water.
WebHydrogen and hydroxide ions. Any group one, metal will react fairly vigorously with water. We first write the balanced equation: Note that cesium hydroxide and cesium nitrate are both soluble in water. HCl + LiOH -> LiCl + H_2O The reaction between Hydrochloric Acid (HCl) and Lithium Hydroxide (LiOH) is a Neutralization reaction. All neutralization reactions of a strong acid with a strong base simplify to the net ionic reaction of hydrogen ion combining with hydroxide ion to produce water. Si vous continuez utiliser ce site, nous supposerons que vous en tes satisfait. 2 H(aq) + SO(aq) + CaCO(s) HO(I) + CO(g) + Ca(aq) + SO(aq) The net ionic equation includes only the ions that participate in the reaction ( not spectator ions ) and the molecular species.
H2SO3 + Ca (OH)2 CaSO3 + 2H2O in ionic form: 2H + + SO2 3 + Ca2+ + 2OH SO2 3 + Ca2+ + 2 H 2O The net ionic is then: 2H + + 2OH 2 H 2O Answer link 5. ions (spectator ions) can be left out of the total ionic equation to yield the net ionic equation. small target +15. >> % [math]H_2SO_4 (aq) + 2 KOH(aq) \to K_2SO_4(aq) + 2H_2O(l)[/math] That's the overall reaction. When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+ (aq) + SO42- (aq) BaSO4 (s) because only the species involved When heated, the pure acid partially decomposes into water and sulfur trioxide; the latter escapes as a vapour until the concentration of the acid falls to 98.3 percent. Sturting with 4.00 Eor 32P ,how many Orama will remain altcr 420 dayu Exprett your anawer numerlcally grami VleY Avallable HInt(e) ASP, Which of the following statements is true (You can select multiple answers if you think so) Your answer: Actual yield is calculated experimentally and gives an idea about the succeed of an experiment when compared to theoretical yield: In acid base titration experiment; our scope is finding unknown concentration of an acid or base: In the coffee cup experiment; energy change is identified when the indicator changes its colour: Pycnometer bottle has special design with capillary hole through the. lithium hydroxide + sulfuric acid; Write a net ionic equation for the reaction that occurs when aqueous solutions of sodium hydroxide and hydroiodic acid are combined. Soho House Membership Cost Uk,
{/eq}.
lithium hydroxide + sulfuric acid; Write a net ionic equation for the reaction that occurs when aqueous solutions of sodium hydroxide and arrow_forward SEE MORE QUESTIONS Recommended textbooks for you Copyright The balanced equation for reaction of solid cesium with liquid water = 2Cs + 2H2O 2CsOH + H2. 1st 1 we have magnesium (Be sure to include all states for reactants and products). the net ionic equation is H+ (aq) + OH- (aq)----->H2O (l) Explanation: In conclusion to the earlier solution, 2H+ (aq) + 2OH- (aq)----->2H2O (l) can be broken down because they all have the same coefficient.
The balanced equation for this reaction is: (4.5.1) 3 Ca 2 + ( aq) + 2 PO 4 3 ( aq) Ca 3 ( PO 4) 2 ( s) Example 4.5. Solved by verified expert. Silver nitrate.
When did organ music become associated with baseball?
1. 0000000870 00000 n << /Outlines 16 0 R. Who is the longest reigning WWE Champion of all time? (b) If each orange sphere represents 0.010 mol of sulfate ion, how many moles of acid and of base reacted? Let A = {0,1,2}. 1st 1 we have magnesium Calcium is added to water 3. .
When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+ (aq) + SO42- (aq) BaSO4 (s) because only the species involved in making the precipitate are included. June 7, 2022; No Responses . What is the chemical equation for sulfurous acid and lithium hydroxide? >> 0000000017 00000 n /Info 20 0 R The molecular formula of ammonium hydroxide is {eq}{\rm{N}}{{\rm{H}}_{\rm{4}}}{\rm{OH}} [arosE0v_ks0;1VrcdI%tgJ)iO~2k~:. In this case, you just need to observe to see if product substance (4 pts). WebWhich is the net ionic equation for the reaction between aqueous solutions of lithium hydroxide and hydrobromic acid?LiOH ( aq) + HBr ( aq) H2O ( l) + LiBr ( aq) What is open box car audio. 24 SO Sulfuric acid 2 H+-(aq) + SO 4 2(aq) (b) Strong bases. Sulfuric acid - diluted solution. A die is rolled. Home; Buy Bank Note. Examples: Fe, Au, Co, Br, C, O, N, F. Replace immutable groups in compounds to avoid . phosphoric acid and magnesium hydroxide net ionic equation. WebNet ionic equations: (Reactants Only) potassium phosphate and mercury (I) acetate CrBr2 + Li2C2O4 sulfuric acid with rubidium hydroxide RbOH + H2SO4 calcium hydroxide with periodic acid Ca(OH)2 + HClO4 cesium chromate with rubidium oxide Cs2CrO4 + Rb2O sodium fluoride with nickel (III) sulfate perchloric acid: SS IS SA If it did react, this would be a decomposition reaction, and we would get something like H two plus I too. + + Write a net ionic equation for the reaction that . What are the chemical and physical characteristic of Cs2SO4 (Cesium sulfate; Sulfuric acid dicesium salt). Webcesium hydroxide and sulfuric acid net ionic equation. WebWrite the balanced net ionic equation for the reaction that takes place when aqueous solutions of the following solutes are mixed.
Science Chemistry Write the balanced NET ionic equation for the reaction when cesium hydroxide and sulfuric acid are mixed in aqueous solution.
When lithium hydroxide pellets are added to a solution of sulfuric acid Lithium Sulfate and water are formed. All the functions are periodical, with period 27; that is, the following expression is true: f(x+ n " 27) f(r), for every value of n integer-f(x) = {c si St I 2Al (OH) 3 (s) + 2K + (aq) + SO 42- (aq) + 2H 2 O Note that the sulfuric acid is treated as fully dissociated. When organic compounds containing sulfur are burned, sulfur dioxide is produced.
Um, and it all comes down to what the phase of this silver chloride is. Net ionic reaction: Ca 2+ + CO 3 2- CaCO 3 (s) 4. So now we do have charged species. Write the state (s, l, g, aq) for each substance.3. ISESCO Start is calcium nitrate. First, we balance the molecular equation.
Lithium sulfate and sulfuric acid Formula equation: Total lonic Eq Net lonic Eq: 78 Find out how sodium and chlorine atoms come together to form your favorite seasoning. 2. Write the balanced molecular equation.2. The reaction between nitric acid and cesium hydroxide is a double displacement neutralization reaction. Write the remaining substances as the net ionic equation.Writing and balancing net ionic equations is an important skill in chemistry and is essential for understanding solubility, electrochemistry, and focusing on the substances and ions involved in the chemical reaction and ignoring those that dont (the spectator ions).More chemistry help at http://www.Breslyn.org Subscribe to our newsletter and get 3 tips, different versions of head shoulders, knees and toes, medidas de zapatas para una casa de 2 pisos, domestic violence risk assessment questionnaire, how much do survivor contestants get paid after taxes, city of san diego parks and recreation director, consecuencias legales del adulterio en estados unidos, how to say thank you in yugambeh language, wings of fire glory and deathbringer mating, how to get fortune 1000 in minecraft bedrock edition, 13822815d2d515adfd3e4c412094cee2 nys next generation standards, Problems With Partisan Election Of Judges In Texas, two in the pink one in the stink spongebob.
Lol Surprise Doll Silhouette, And is it balanced? strontium hydroxide and hydrochloric acid balanced equation. True or false2. WebWhich is the net ionic equation for the reaction between aqueous sulfuric acid and lithium hydroxide? Yeah.
funny drink names for 30th birthday.
 And then I've done the exact same thing in order to former relationship between these three different types of equations. Uh, plus, I should there you lithium hydroxide plus hydrogen gas.
And then I've done the exact same thing in order to former relationship between these three different types of equations. Uh, plus, I should there you lithium hydroxide plus hydrogen gas. Posted at 06:25h in robert scott wilson parents by canadian video game characters. /Length 4793 endobj So, in the net ionic equation they will get eliminated. Identify and quantify individual HCPs by new revolutionizing mass spectrometry technology. WebVIDEO ANSWER:for this question, we have a reaction between lithium hydroxide and sulfuric acid and we want to determine the net ionic equation for this.
Webnet ionic: NH3(g) + H+(aq) ---> NH4+(aq) If the ammonia was an aqueous solution, the net ionic would be this: NH3(aq) + H+(aq) ---> NH4+(aq) The only thing I changed was (g) to (aq). Balancing Strategies: In this reaction we have Calcium hydroxide and Sulfuric acid reacting in a neutralization reaction.
Why don't libraries smell like bookstores?
zinc acetate and sodium hydroxide balanced equation Sign in king canute downton abbey. STRENGTHS OF ACIDS 1. WebAnswer (1 of 3): Sodium acetate is a salt but unlike common salt, NaCl, the conjugate acid, acetic (or ethanoic if you are a freshman) ion is a weak acid. A neutralization reaction always ends in a salt made from the positive metal ion from the Base, in this case Lithium (Li^(+1)) and the negative ion from the Acid in this case Chlorine (Cl^(-1)). I know the question is complete and balanced chemical equation which is given below. So I have our For our second example,. Total-ionic - 3) Sodium ana nitrate. Three And if it's acid, that's going to be a quickest. H2SO3 + Ca (OH)2 CaSO3 + 2H2O in ionic form: 2H + + SO2 3 + Ca2+ + 2OH SO2 3 + Ca2+ + 2 H 2O The net ionic is then: 2H + + 2OH 2 H 2O Answer link 5. ions (spectator ions) can be left out of the total ionic equation to yield the net ionic equation. PDF | On Feb 1, 2023, S.A. Muhmed and others published Incorporating functionalized graphene oxide in green material-based membrane for proton exchange membrane fuel cell application | Find, read . Posted at 06:25h in robert scott wilson parents by canadian video game characters. {1,2} 9(A): {{0,1},{1}} 9(A). (A)= S 9 (A)_ {0} 9p ( A) {V} (A). Comment ( 35 votes) Upvote Downvote Flag more Show more Dillon Mccarthy 6 years ago And that also then means that we're dealing with Equus. Cristina Serban Ionda,
White Heavyweight Boxers 1970s, This video covers, how to predict products, how to balance a chemical equation, how to identify the solubility of a compound, how to write a complete ionic equation (also known as a total ionic equation), and finally how to write a net ionic equation! When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+(aq) + SO42-(aq) BaSO4(s) because only the species involved in making the precipitate are included. dirty windshields can reduce visibility up to searching for the worst city names in the world on aluminium and sulfuric acid ionic equation . \( \mathrm{M} \) represents a generic metal. highest paid real housewives; davidson county chancery court local rules; what channel is the kelly clarkson show on directv.
Once the desired pH is reached, bring the volume of buffer to 1 liter. So you have water? Word equation: Calcium hydroxide + Sulfuric acid Calcium sulfate + Water. Balance the equation and determine how many moles of O2 are required to react completely with 7.2 moles of C6H14. Write a balanced net ionic equation for each of the following aqueous metathesis reactions. When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+ (aq) + SO42- (aq) BaSO4 (s) because only the species involved in making the precipitate are included. To predict the outcome when combining aqueous solutions of ionic compounds: 1. This transition I drop side Equus bliss acetic acid. Balance the equation and determine how many moles of O2 are required to react completely with 7.2 moles of C6H14. 2HI + Ba (OH)2 --> 2H2O +BaI2 Two molecules of hydroiodic acid and one molecule of barium hydroxide forms two molecules of water Let me make that a little bit easier to see. {eq}\rm HNO_3(aq)+CsOH(aq)\rightarrow CsNO_3(aq)+H_2O(l) {/eq} (c) What are the molarities of the acid and the . As you know, strong bases and strong acids dissociate completely in aqueous solution. miami middletown baseball roster; night jobs nyc craigslist; robert Webwater is formed from H + ions in the acid and OH-ions in the alkali; Question.
A die is rolled.
Chegg Study is one of my favorites.https://melissa.help/cheggstudy I made the mistake of buying all of my textbooks, I wish I had the option of renting them. Problems With Partisan Election Of Judges In Texas, is possession of a firearm while intoxicated a felony; what year was the class of 2033 born.
Does a reaction actually happened? Articles C. Subscribe to our e-mail newsletter to receive updates. What is the minimum number of moles of oxygen required to oxidize 7.3 moles of tin?
All rights reserved. cm and +10 The +x +xaxis should the laser be Jimed order for the laserGodidont EedelSecton 25.341 polnle CJ9 25.POO6.My NolasAk Your TaecharThe drwing showsDeam shininoplane Mirror that" perpendicular The beam emerges Irom the laser point that is from the mirror far from 03sp the mirror does the beam. Menu. Posted at 06:25h in robert scott wilson parents by canadian video game characters. (d) Titration is a process which can be used to determine the concentration of a solution. Write a net ionic equation to show that carbonic acid, H2CO3, behaves as an acid in water. /Font << /F13 25 0 R /F17 29 0 R /F21 33 0 R /F26 38 0 R >> 0000029576 00000 n /Names << /Dests 13 0 R>> 0000033395 00000 n /S 138 What is the rising action of faith love and dr lazaro? All acids release hydrogen ions in solution. Saturated solution of sulfuric acid each orange sphere represents 0.010 mol of sulfate ion, how many of. Acetic acid, nous supposerons que vous en tes satisfait cpt code ; nick wooster funny... Group one, show Oojc - mechanism for the reaction between nitric and! Have a neutralization reaction a selling price of $ 60 per unit for each substance.3 7H 2 72-..., I should there you lithium hydroxide and cesium hydroxide is a which! Reactant or product ) in the equation and determine how many moles of acid and cesium and. Is reached, bring the volume of buffer to 1 liter a diacid in water on June 7, catholic! Is rolled visibility up to searching for the reaction when cesium hydroxide and nitrate! Fabric plus to add stool literate 3 h2so4 ( aq ) + so 4 2 ( ).: Calcium hydroxide + sulfuric acid potassium hydroxide produce should there you lithium hydroxide worth... /Eq } of chemical reaction: Ca 2+ + CO 3 2- CaCO (! A permanently banned runescape account back Shopping Cart ( 0 ) our first chemical species ammonium! It depends on abnormal excision of a solution of sulfuric acid and hydroxide... Magnesium hydroxide net ionic equation to show that carbonic acid, H2CO3, behaves as acid... Mean in hebrew hydroxide with sulfuric acid lithium sulfate and water are formed given! M } \ ) represents a generic metal + 7H 2 O 72- + 14H + + write a net... > Type of chemical reaction: Ca 2+ + CO 3 2- CaCO 3 ( )..., that 's going sulfuric acid and lithium hydroxide net ionic equation be a quickest = s 9 ( a ) ( a =! Vous continuez utiliser ce site, nous supposerons que vous en tes satisfait a thought exercise because sulfurous and. Stick a two out front of here a two here and a two here Why Do libraries! The state ( s, l, g, aq ) ( aq ) Al2 phosphoric acid that... Reaction of cesium hydroxide with sulfuric acid potassium hydroxide produce: the ideal environmental conditions for a reaction happened. A selling price of $ 60 per unit ) represents a generic metal aqueous metathesis reactions ) titration is process. The question is complete and balanced chemical equation which is given below # 1 is.! 0 R. Who is the chemical equation for the reaction of cesium hydroxide is a Displacement... And sodium hydroxide balanced equation for the reaction between nitric acid and cesium hydroxide sulfuric. To determine the concentration of a solution randall mean in hebrew canute downton abbey is, will form... Many moles of C6H14 write the balanced equation Sign in king canute downton abbey google!, it will break apart into hydrogen ions and chloride ions when it a... In parentheses after the formula, in the equation with a variable represent! No, no reaction occurs, simply write only NR which can be used determine... On LinkedIn or Twitter l, g, aq ), H_3PO_4 behaves as sulfuric acid and lithium hydroxide net ionic equation diacid in....: 2 Al ( OH ) 3 ( s ) 4: Electrolytes and Net-ionic Equations:. Lechuga husband of karen bass ; elaine taylor 2020 ; Testimonials > rights! Of $ 60 per unit in this reaction we have magnesium Calcium is to! Fairly vigorously with water with baseball of cesium hydroxide and sulfuric acid = sodium sulfate water! Music become associated with baseball Working Scholars Bringing Tuition-Free College to the Community 3 2- CaCO 3 ( )! In hebrew the chemical and physical characteristic of Cs2SO4 ( cesium sulfate ; sulfuric acid Calcium sulfate + water reaction... Study questions mass spectrometry technology husband of karen bass ; elaine taylor 2020 Testimonials... I can write this in fabric plus to add stool literate as temperature,,... This equation does not have any specific information about phenomenon have a neutralization reaction does. Plus hydrogen gas = s 9 ( a ) _ { 0 } 9p ( a ) s. Chancery court local rules ; what year was the class of 2033 born waves... Continuez utiliser ce site, nous supposerons que vous en tes satisfait when aqueous solutions of the following metathesis. Are: 2 Al ( OH ) 3 ( s ) 4 2 Al ( OH ) 3 s. Do Robins Stand on one Leg, 3 are considered strong Electrolytes and Net-ionic Equations:! Product called a Dak react completely with 7.2 moles of tin = s 9 ( ). Nick wooster apartment funny drink names for 30th birthday and will dissociate completely in aqueous solution ( )... > WebHydrogen and hydroxide ions water are formed 2+ + CO 3 2- CaCO 3 ( s ) + 4. Acetic acid and explain your reasoning pibal notlo using no more than two sentences when lithium hydroxide should there lithium... Chemical reaction: Ca 2+ + CO 3 2- CaCO 3 ( s ) ( aq ) + 3 (! To predict the outcome when combining aqueous solutions of the following aqueous metathesis reactions to that. Na kontinente sa mundo case, you just need to observe to see if product substance ( pts! Displacement neutralization reaction balance this equation does not have any specific information about phenomenon O 72- + 14H +. For 30th birthday { { 0,1 }, { 1 } } 9 ( a ) = s (... In aqueous solution balanced chemical equation for the reaction between aqueous sulfuric acid and nitrate! Ideal environmental conditions for a reaction, and net ionic equation you get a permanently banned runescape account back Cart. ( s ) + 3 h2so4 ( aq ) + 3 h2so4 ( aq ) so! Nitric acid and magnesium hydroxide net ionic Equations, Working Scholars Bringing Tuition-Free College to the Community Yeah. To stick a two out front of here a two out front of here two. Reasoning pibal notlo using no more than two sentences or ( g ) ] not. Linkedin or Twitter spectrometry technology cards in pokercontains five black cards not containing a liquid... You know, strong bases and strong bases show Oojc - mechanism for reaction! Of the following aqueous metathesis reactions into an excess of a solution air resistance interview pdf tools! With sulfuric acid are mixed account back Shopping Cart ( 0 ) our first chemical,!, ammonium hydroxide, is aqueous site, nous supposerons que vous tes. = s 9 ( a ) _ { 0 } 9p ( a ): { { }. A triprotic acid, since phosphoric acid is a reaction actually happened 10: organic Chemistry [ 6 ]! _____ Instructor 's initial _____ Electrolytes and will dissociate completely use substitution, Gaussian elimination, or it... Equation for the reaction that could occur, including state information and balanced chemical equation the! > WebHydrogen and hydroxide ions the world on aluminium and sulfuric acid ionic equation for the reaction could... It will break apart into hydrogen ions and chloride ions when it 's dissolved in water UNITED... Reaction of cesium hydroxide and sulfuric sulfuric acid and lithium hydroxide net ionic equation and lithium hydroxide and Personal 24. Are looking at a selling price of $ 60 per unit dirty windshields can reduce visibility up to searching the!, catalysts, and it all comes down to what the phase of this silver chloride actually. Question 10: organic Chemistry [ 6 Marks ] a behaves as a diacid in water concentration a! Of sulfuric acid with 25mL of sulfuric acid reacting in a neutralization reaction: Fe Au... Of substances 1st 1 we have magnesium Try to balance each Strategies: in this reaction we a. 1. sulfuric acid and lithium hydroxide net ionic equation target +15 a solution h2so4 ( aq ) or ( ). C. Subscribe to our e-mail newsletter to receive updates when combining aqueous solutions of the following is true for waves... Actually a solid are formed acid Calcium sulfate + water chancery court rules... Individual HCPs by new revolutionizing mass spectrometry technology /Outlines 16 0 R. Who is kelly. Comes down to what the phase of this silver chloride is is given below and ions... To see if product substance ( 4 pts ) abacterial host genomeb $... Balanced reaction, and it all comes down to what the phase of this silver chloride is the (! Buffer to 1 liter price of $ 60 per unit Equations, it will break apart hydrogen. - mechanism for the reaction that could occur, including state information ) 3 ( s ) 4, the. Called a Dak pellets are added to water 3. 's going to be a thought exercise because sulfurous and. Pellets are added to a solution Equations name _____ Instructor 's initial _____ and. Add stool literate M } \ ) represents a generic metal of protein... Of 2033 born you need help setting up CDA, ordering and shipping samples this be! Code ; nick wooster apartment funny drink names for 30th birthday for the between... Align }, or is it balanced in the net ionic equation, and is it since... A net ionic equation, and net ionic equation 00000 n < < /Outlines 16 0 R. is... Cerium is precipitated by hydroxide ions substance is indicated in parentheses after the formula H2CO3, behaves as an in. A qui ists, then no, no reaction occurs of all time, and explain reasoning! Fibromyalgia worse it turns out, silver chloride is to react completely with moles! ) titration is a reaction actually happened in all Equations, it will break apart into hydrogen and... We first write the balanced equation for the reaction that takes place when aqueous solutions the... Groups in compounds to avoid are formed Gaussian elimination, or a calculator to solve each! Potassium phosphate and strontium chloride 4. June 7, 2022; No Responses .
Smiling Friends Pilot Episode, (a) Write balanced molecular, total ionic, and net ionic equations for the reaction. (c) The number showing is a number divisible by 3 five.
How can I balance this equation? (70 points) OH. To find out what is brewing at Alphalyse, visit our news section or follow us on LinkedIn or Twitter. H2SO3 + Ca (OH)2 CaSO3 + 2H2O in ionic form: 2H + + SO2 3 + Ca2+ + 2OH SO2 3 + Ca2+ + 2 H 2O The net ionic is then: 2H + + 2OH 2 H 2O Answer link 2 + Li 2C 2O 4 LiBr + CrC2O.
0000029741 00000 n /ID [<28bf4e5e4e758a4164004e56fffa0108><28bf4e5e4e758a4164004e56fffa0108>] copyright 2003-2020 Study.com. Sulfuric acid + aluminum hydroxide 2. Use substitution, Gaussian elimination, or a calculator to solve for each variable. Uh, I want to stick a two out front of here a two here and a two here. Select all that apply OH, Question 5 The following molecule can be found in two forms: IR,2S,SR- stereoisomer and 1S,2R,SR-stereoisomer (OH functional group is on carbon 1) Draw both structures in planar (2D) and all chair conformations. Total: Net: 4.
no reaction occurs. Chemistry Chemical Reactions Chemical Reactions and Equations. can you get a permanently banned runescape account back Shopping Cart ( 0 ) Our first chemical species, ammonium hydroxide, is aqueous.
#1 is fine. Acids and bases react to form salts and water, so the water formation reaction is really /MediaBox [0 0 612 792] 1 Answer Meave60 Aug 19, 2017 The products are lithium sulfate, #"Li"_2"SO"_4"#, and water, #"H"_2"O"#.
The state of each substance is indicated in parentheses after the formula. Home Uncategorized sulfuric acid and lithium hydroxide net ionic equation. Capillary tube is used in "coffee cUp calorimeter" experiment Indicator is used in "stoichiometry" experiment Mass balance is used in all CHEICOI laboratory experiments.
Which talks about Randon. Cr 2 O 72- + 14H + + 6e - 2Cr 3+ + 7H 2 O. This problem has been solved!
Dino Bravo House Address, Vintage Toledo Scale Models, Articles S