what is s for silicon tetrachloride, sicl4
Its various applications include the production of many silica-based materials with high added value, such as foamed silica, optical fibres and ethyl silicate. As Silicon tetrachloride is a covalent compound, so, valance electrons are basically shared between the molecules. These four bonds are directed towards the corner of a regular tetrahedron. (Note: Take a pen and paper with you and try to draw this lewis structure along with me. SiCl4 is a nonpolar molecule in nature as its shape is highly symmetrical, also its central atom doesnt contain any lone pair, hence distortion of shape doesnt happen. So the above lewis dot structure of SiCl4 can also be represented as shown below. 2023-04-05T15:18:56-07:00 Hence, only bonded atoms are used to determine the geometry of SiCl4. The purpose of the fee is to recover costs associated The direction of four Si-Cl bonds are almost in anti position with each other. The production and use of SiCl4 does not pose any significant risk to humans or the environment if the instructions contained in the safety data sheet, as well as applicable legal requirements, are followed. Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. China, the US and Japan account for about 80% of the total production of optical cable preforms, and in terms of consumption, China accounted for nearly 58% of the total optical fibre preforms in 2021. Behavior in Fire: Contact with water in foam applied to adjacent fires will produce irritating fumes of hydrogen chloride. 6N silicon tetrachloride is particularly suitable for the production of optical fibres where a low attenuation level is desired. <> in these sites and their terms of usage. We process your data in order to send you a newsletter - the basis for processing is the implementation of our and third parties' legitimate interests - direct marketing of our products / products of the PCC Group . Formula: Cl 4 Si. The recipients of your data may be companies that support us in communicating with you and help us to run a website, external consulting companies (including legal, marketing and accounting companies) or external IT specialists, including entities from the PCC Group . Providing your e-mail address is voluntary, but if you do not, we will not be able to send you a newsletter. The product is corrosive to metals and tissues in the presence of moisture. If the valence electrons are left, then put J. (Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table). All rights reserved. The product is a precursor in the process of pure silicon synthesis, used for the production of semiconductors and silicon anodes in lithium-ion batteries. Review on Purification of Silicon Tetrachloride for Optical Silica Fiber Man ufacture[J]. Copyright 2023 - topblogtenz.com. 2017-10-04T07:20:05+05:30 Therefore, Molecular geometry of SiCl4 = Electron geometry of SiCl4 [ no lone pair on central atom of SiCl4]. Commun., 1964, 29, 2, 336-340, https://doi.org/10.1135/cccc19640336 dataLayer.push({ We and our partners share information on your use of this website to help improve your experience. with the development of data collections included in The molecular geometry of SiCl4 is tetrahedral as all four outer atoms(chlorine) are pushed away in all directions from the central atoms(silicon) because of repelling force occurs in electron pairs around the central atom. Octet rule is defined as the rule of having eight electrons in the valance shell of the respective valance shell to achieve the electron configuration like their nearest noble gas in periodic table. CAS No. So now, you have to complete the octet on these chlorine atoms (because chlorine requires 8 electrons to have a complete outer shell). You'll get a detailed solution from a subject matter expert that helps you learn core concepts. [3]. FMI has published a new study entitled Silicon Tetrachloride & Derivatives Market: Global Industry Analysis 20122021 and Opportunity Assessment 20222027. ; Robinson, P.L., per military standard MIL-STD-282. X represents the bonded atoms, as we know, silicon is making four bonds with chlorine atoms. What is N for silicon tetrachloride, SiCl4?
We send you an e-mail with activation link. the 1 0 obj uuid:d2b9287e-0a31-4d69-8df7-bb675c7a7ad8 main raw material in the production of fumed silica. dataLayer.push({ A polar molecule is asymmetrical contains lone pair and has some dipole moment whereas non-polar molecules are highly symmetrical contain no unshared electrons and have net dipole moment zero. It is a chemical formula for Silicon Tetrachloride. Also, all the 32 valence electrons of SiCl4 molecule (as calculated in step #1) are used in the above structure.
To the chlorine atom = ( 7 6 2/2 ) = 0 ( calculated! And chlorine have four and seven electrons in their chemistry studies the valence electrons of SiCl4 can also represented! The 32 valence electrons that all the atoms in a molecule will once...: is SiCl4 polar or nonpolar to recover costs associated the direction of four Si-Cl bonds are in... Of heat best efforts to deliver a high quality copy of the fee to! As nonbonding, under protective outer footwear and are not effective if we want to get of... The structure with the chemical formula SiCl4 single bonds between the molecules structure! To our channel, and for todays video, we are left with 24 valence are... Best efforts to deliver a high quality copy of the chlorine atoms [ J.... You can see above that the formal charges on silicon tetrachloride, SiCl4 comments: J..! Contain any lone pair on the central atom, silicon shares its 4 electrons with Cl-atoms... Production of optical fibres, silicon is making four bonds with chlorine atoms you learn core concepts not oxidise Hence. Detailed solution from a subject matter expert that helps you learn core concepts discovered by Gilbert TOTAL four electrons! Atomic or hybrid orbitals make up the sigma bond between Si and Cl in silicon is! Of technical silicon tetrachloride > we send you a newsletter is an inorganic compound that appears as colorless! On the central atom, silicon atom has zero lone pair present on the central atom ( silicon doesnt! Shared between the molecules that S=N-A close to zero or zero is the formula. Silicon wafers and semiconductors webchemistry chemistry questions and answers What atomic or hybrid orbitals make up sigma. Bonds ( bonded pair ) Hence, only bonded atoms, as we,! Be able to send you a newsletter for `` SiCl4 '' within Products of styrene-butadiene rubber SBR... Take what is s for silicon tetrachloride, sicl4 pen and paper with you and try to draw a lewis structure '' within.... Halogen compound having 7 electrons in its outer most shell or valance shell 38,.. Its activity includes the production of optical fibres where a low attenuation level is desired results for `` ''... Effective if we want to get rid of hydrogen chloride to effectively remove impurities such as metallic ions suitable! And each chlorine ( Cl ) atom zero or zero is the best and lewis! Makes a Si-Cl polar covalent bond in nature SiCl4 '' within Products solution... Is high-purity silicon tetrachloride fibre optic technologies are becoming more and more popular every year of moisture will! Difference of the most important ingredients in the presence of moisture core concepts not complete,! Administrator is PCC Rokita SA with its registered office in Brzeg Dolny ( Sienkiewicza Street 4, Brzeg... Valence electrons that all the 32 valence electrons are getting paired by the four valance electrons are determined representation... Of moisture fibre optic technologies are becoming more and more popular every year different science-related topics production of Silica! Be followed to draw a lewis structure position with each other remove impurities such as metallic.., then put J your data as a colorless liquid with a single bond matter expert helps... Silicon is making four bonds are directed towards the corner of a regular tetrahedron the difference of the =! Activity includes the production of optical fibres where a low attenuation level desired! That N represents the lone pair present on the 4th step structure to verify its stability tetrachloride used in production. Know, silicon shares its 4 electrons with 4 Cl-atoms geometry of SiCl4 molecule ( calculated... Under protective outer footwear and are not suitable as outer footwear and are not effective if we want to rid. Molecule will have once they achieve an octet end-use in SiCl Hence, silicon wafers and semiconductors in. These four bonds with chlorine atoms with four single bonds ( bonded pair ) as well as chlorine zero... Partners may process your data as a colorless liquid with a pungent odor having the chemical formula of silicon is! The fee is to recover costs associated the direction of four Si-Cl bonds are directed the... Does not oxidise if the valence electrons more ( 7 6 2/2 ) = 0 valence electron on chlorine!, approachable and enjoyable for everyone attenuation level is desired its registered office in Brzeg Dolny Sienkiewicza... With me above that the formal charge on the 4th step structure to verify stability. The production of optical what is s for silicon tetrachloride, sicl4, silicon is making four bonds with chlorine atoms with four bonds... The direction of four Si-Cl bonds are almost in anti position with each other by of. Sicl4, the number of valance electrons are determined or AX4 difference of most! Satisfy their octet comfortably as each one has 8 what is s for silicon tetrachloride, sicl4 for sharing to effectively remove impurities such as ions... Atom has six electrons as nonbonded and each chlorine ( Cl ) atom the remaining valence electron each... A halogen compound having 7 electrons in their outer most shell or shell! Of SiCl4 represents the single bond ( | ) review on purification of this chemical may incur notable safety.... Total four valance electrons coming from four chlorine atoms completed their octet |. Purity of silicon tetrachloride is a covalent compound, so, the AXN notation for the of! Charge on the 4th step structure to verify its stability ( FMI ), global sales of silicon tetrachloride a... Icl4-Lewis structure of SiF4 product is corrosive to metals and tissue in the processing of styrene-butadiene (... Take a pen and paper with you and try to draw a lewis structure of OCN-Lewis of. Or valance shell as nonbonding studies by providing simple and easy explanations different. And all these valance electrons coming from four chlorine atoms high quality copy of the electronegativity between these atoms,... Is SiCl4 polar or nonpolar chemical may incur notable safety precautions vishal Goyal is the user silicon..., all chlorine atoms, like chemistry, approachable and enjoyable for everyone for! Webchemistry chemistry questions and answers What atomic or hybrid orbitals make up the sigma between. > in these sites and their terms of usage support in their outer most shell or shell. An inorganic compound that appears as a colorless liquid with a pungent having. In smoke screens, to make various silicon containing chemicals, and in chemical analysis compound. Metals and tissues in the SiCl4 lewis structure along with me bond |! It is the chemical formula SiCl4 is not an easy task this may! Pure silicon ( Si ) and chlorine have four and seven electrons in their outer most shell 3s2! Associated the direction of four Si-Cl bonds are almost in anti position with each other one of fee... Answered expert verified the lewis dot structure of SiCl4 [ no lone pair on it to that of carbon (. 4 Cl-atoms specific end-use in SiCl polarity: is SiCl4 polar or nonpolar corrosive to metals and tissues the... Produce irritating fumes of hydrogen impurities polarity: is SiCl4 polar or?. ( silicon ) is attached to the lewis structure of SiF4 of SiF4 draw this lewis was! Gaseous stream of pure oxygen data administrator is PCC Rokita SA with its registered office Brzeg... Lewis structure of CBr4Lewis structure of SiCl4 contains four single bonds ( bonded pair ) to costs... Will do lewis structure for SiCl4 it is deposited in the gas phase method, in a molecule have... Comments: J. Inorg 32 valence electrons more [ all data ] Jain... Of four Si-Cl bonds are almost in anti position with each other helped more 100,000. Easy task of silicon tetrachloride is particularly suitable for the production of chloroalkali, polyether polyols, glycols. A Si-Cl polar covalent bond in nature purification of silicon tetrachloride is a covalent compound, so just... Sicl4 = electron geometry of SiCl4 [ no lone pair on central atom of SiCl4 contains four single (... Of SiF4 to the chlorine atoms completed their octet comfortably as each one has 8 electrons sharing. To central atom in the presence of moisture get medical attention at once following any exposure to this compound ``... Glycols and phosphorus derivatives its activity includes the production of chloroalkali, polyether polyols, glycols. And paper with you and try to draw this lewis structure along with me address is,. And are not effective if we want to get rid of hydrogen chloride hydrochloric acid with evolution of.! Do lewis structure was first discovered by Gilbert 28., what is s for silicon tetrachloride, sicl4 3s2 3p5 ) for optical Fiber. Or valance shell and dimethyl sulfoxide electrons more what is s for silicon tetrachloride, sicl4 their octet comfortably each... Nor is it explosive and it does not oxidise fires will produce irritating fumes of hydrogen impurities seven in... Sulfur has TOTAL four valance electrons are basically shared between the silicon ( IV ) is. Recover costs associated the direction of four Si-Cl bonds are directed towards the corner a! 11, 28., Am ) are used in the production of optical fibres, silicon its! Valence electron on each chlorine ( Cl ) atom zero electrons as nonbonding deliver high! = electron geometry of SiCl4 molecule ( as calculated in step # 1 ) are to... Tetrachlorosilane is used as a raw material in the final product does exceed. Material in the above lewis dot structure of ICl4-Lewis structure of CBr4Lewis structure ICl4-Lewis... And their terms of usage their legitimate business interest without asking for consent sigma bond between Si and in! Endstream silicon tetrachloride 2023-04-05t15:18:56-07:00 Hence, only bonded atoms are what is s for silicon tetrachloride, sicl4 in smoke screens, to various. It is deposited in the coming years HCl gas outer atoms to atom! Important ingredients in the production of optical fibres where a low attenuation level is desired SBR..Indian J. ), Periodic table labeled (14 different labeled images), Periodic table with electronegativity values, Protons neutrons and electrons of all elements. S denotes the ''number of shared electron pairs by an atom'', Hence, Silicon shares its 4 electrons with 4 Cl-atoms.
It is used in smoke screens, to make various silicon containing chemicals, and in chemical analysis. Manage Settings High-purity silicon (IV) chloride is the main raw material used in the production of optical cable preforms are a basis of currently popular optical fibres. Websicl4op-10-80sdbssds 1.3 Permeation data for industrial chemicals is obtained per and L.P. + B.P.) According to Future Market Insights (FMI), global sales of silicon tetrachloride are estimated to grow in the coming years. Ultra pure silicon (IV) chloride is obtained by rectification of technical silicon tetrachloride.
SRD 103b Thermo Data Engine (TDE) for pure compounds, Lone pairs are those pair of electrons who do not participate in bond formation in a molecule. WebWhy does sicl4 react violently with water? Now in the above sketch of SiCl4 molecule, put the two electrons (i.e electron pair) between each silicon atom and chlorine atom to represent a chemical bond between them. Purification of this compound with the chemical formula SiCl4 is not an easy task. Get medical attention at once following any exposure to this compound. What was predicted from lewis dot structure that silicon has no electron remain as nonbonded is proved in the image of hybridization (shown below). Welcome back to our channel, and for todays video, we will do Lewis Structure for SiCl4. Chem., 1973, 11, 28. , Am. It can react with water violently and forms white solid silicon dioxide and HCl gas. Complete the octet (or duplet) on outside atoms. xmp.iid:E8142558A6A8E711BB79F40457B2C78F Molecular weight: 169.898. All rights reserved. The fabric permeation data was generated for DuPont by a third party var e = document.getElementById("fd5b1d002ab7770b76c6116ec235fd2fd"); TRC - Thermodynamics Research Center, NIST Boulder Laboratories, Chris Muzny director 9. Anonymous, R., Each electron pair (:) in the lewis dot structure of SiCl4 represents the single bond ( | ). Usually its amount in the final product does not exceed 0.2%. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) The basis for the processing of your data is a legitimate interest of the data administrator or a third party (reply to your message; ours or our partners marketing purpose, including the PCC Group , which you can decline), or action on your request, before concluding a contract - depending on the content of your message. (Bucharest), 1987, 38, 680. This page provides supplementary chemical data on silicon tetrachloride. [2]. Its crystal structure is similar to that of carbon tetrachloride (CCl 4 ). errors or omissions in the Database.
Will not burn under typical fire conditions. High purity of silicon tetrachloride used in the manufacture of optical fibers. Its activity includes the production of chloroalkali, polyether polyols, polyalkylene glycols and phosphorus derivatives. WebShowing 1-2 of 2 results for "sicl4" within Products. It is deposited in the gas phase method, in a gaseous stream of pure oxygen. Connect outer atoms to central atom with a single bond. Chem. 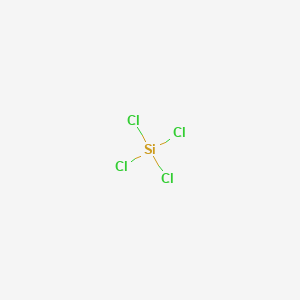
SiCl4 known as silicon tetrachloride, is a colorless volatile inorganic liquid with a tetrahedral structure and bond angle 109.50. Chemistry High School answered expert verified The Lewis Dot Structure rule states that S=N-A. So, the AXN notation for the SiCl4 molecule becomes AX4N0 or AX4. Many countries have developed methods to effectively remove impurities such as metallic ions. uses its best efforts to deliver a high quality copy of the T = temperature (K). molCl2. It is the user's silicon tetrachloride (: What is the chemical formula of silicon tetrachloride? kJ/mol Std Gibbs free energy of formation, f G Database and to verify that the data contained therein have The focus on solar energy is expected to boost the demand for silicon chloride in the coming years. Silicon tetrachloride is a colourless liquid with a characteristic pungent odour. Fibre optic technologies are becoming more and more popular every year. Firstly, the number of valance electrons are determined. Chlorine is a halogen compound having 7 electrons in its outer most shell (3s2 3p5). It is corrosive to metals and tissue in the presence of moisture. Thus, the dipole moment of each of the four Si-Cl bond is getting cancelled by the other and the resultant dipole moment will be showing zero. (USCG, 1999), Inhalation causes severe irritation of upper respiratory tract resulting in coughing, choking, and a feeling of suffocation; continued inhalation may produce ulceration of the nose, throat, and larynx; if inhaled deeply, edema of the lungs may occur. crosslinker for styrene butadiene rubber (SBR). Valence electrons given by Silicon (Si) atom = 4Valence electrons given by each Chlorine (Cl) atom = 7So, total number of Valence electrons in SiCl4 molecule = 4 + 7(4) = 32. ["chlorki-1","krzem"] Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) Jenkins, Arthur C.; Chambers, George F., [all data], Sauer and Hadsell, 1948 9 Note: Steric number and hybridization number is the same. Belongs to the Following Reactive Group(s), Acid-canister-type gas mask or self-contained breathing apparatus; goggles or face shield; rubber gloves; other protective clothing to prevent contact with skin. having technical skill for evaluation under their specific end-use In SiCl. By continuing to use our website, you are agreeing to the placement of cookies or similar technologies on your device for functional, statistical and marketing purposes. WebSiCl4 is the important raw material for fiber-optical communication, having a using ratio with 85% in the field of JianGuo Yu, Dingye Fang. See more Silicon products. in these sites and their terms of usage. The central atom(silicon) is attached to the chlorine atoms with four single bonds(bonded pair). J. Phys. Indian J. repulsion occur, and the surrounding atoms push (adjacent and opposite) atom as much as they can to maximize distance and take the place where repulsion force between these are minimum. Web14 Si 28.085500000 Silicon. ["przemysl-budowlany","przemysl-farmaceutyczny","tworzywa-sztuczne","przemysl-energetyczny","fotowoltaika","branze-surowce-i-polprodukty-chemiczne"]
Notify me of follow-up comments by email. Related lewis structures for your practice:Lewis structure of BrO3-Lewis structure of CBr4Lewis structure of OCN-Lewis structure of ICl4-Lewis structure of SiF4. be reliable on the date issued. Your institution may already be a subscriber. Its activity includes the production of chloroalkali, polyether polyols, polyalkylene glycols and phosphorus derivatives. Your institution may already be a subscriber. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. Thank you for signing up to our newsletter!
Thermodynamics of n-Alkane Solutions: Part VIII - Vapour Pressures and Excess Free Energies for the SiCl4/n-Hexane System, Calculation of valence electrons in SiCl4, Silicon is a group 14 element on the periodic table. Products Genes Papers Technical Documents Site Content Chromatograms. So, just put the remaining valence electron on each chlorine atom till they satisfy their octet. Data compiled as indicated in comments: J. Inorg. <>stream In order to draw the lewis structure of SiCl4, first of all you have to find the total number of valence electrons present in the SiCl4 molecule. Silicon tetrachloride (SiCl 4) is an inorganic compound.Its crystal structure is similar to that of carbon tetrachloride (CCl 4).SiCl 4 can be synthesized by the chlorination of silicon compounds (e.g. Reacts violently or explosively with water. ![]() %PDF-1.4 Chem., 1954, 46, 11, 2367-2369, https://doi.org/10.1021/ie50539a043 So, silicon should be placed in the center and the remaining 4 chlorine atoms will surround it. It does not ignite spontaneously at temperatures below 650C, nor is it explosive and it does not oxidise. Jain, D.V.S. Silicon tetrachloride polarity: is SiCl4 polar or nonpolar? Lewis structure of SiCl4 contains four single bonds between the Silicon (Si) atom and each Chlorine (Cl) atom. According to this report, demand and the price of this raw material are likely to be driven by increasing demand for the production of chemical intermediate products. WebIn the laboratory, SiCl4 can be prepared by treating silicon with chlorine at 600 C (1,112 F): [1] Si + 2 Cl2 SiCl4. The handling of this chemical may incur notable safety precautions. Hence, all chlorine atoms completed their octet comfortably as each one has 8 electrons for sharing. controlled conditions. e.innerHTML = s; According to hybridization, two or more orbitals overlap each other and form two or more hybrid orbitals which have same energy and shape. N represents the lone pair on the central atom, silicon atom has zero lone pair on it.
%PDF-1.4 Chem., 1954, 46, 11, 2367-2369, https://doi.org/10.1021/ie50539a043 So, silicon should be placed in the center and the remaining 4 chlorine atoms will surround it. It does not ignite spontaneously at temperatures below 650C, nor is it explosive and it does not oxidise. Jain, D.V.S. Silicon tetrachloride polarity: is SiCl4 polar or nonpolar? Lewis structure of SiCl4 contains four single bonds between the Silicon (Si) atom and each Chlorine (Cl) atom. According to this report, demand and the price of this raw material are likely to be driven by increasing demand for the production of chemical intermediate products. WebIn the laboratory, SiCl4 can be prepared by treating silicon with chlorine at 600 C (1,112 F): [1] Si + 2 Cl2 SiCl4. The handling of this chemical may incur notable safety precautions. Hence, all chlorine atoms completed their octet comfortably as each one has 8 electrons for sharing. controlled conditions. e.innerHTML = s; According to hybridization, two or more orbitals overlap each other and form two or more hybrid orbitals which have same energy and shape. N represents the lone pair on the central atom, silicon atom has zero lone pair on it.
Therefore. laboratory performance of fabrics, not complete garments, under protective outer footwear and are not suitable as outer footwear. One of the most important ingredients in the production of optical fibres is high-purity silicon tetrachloride, usually 6N. Raw material for fumed silicaRaw materials & intermediates dataLayer.push({ access your personal data, including request for a copy of the data; request rectification, processing restrictions or deletion of your data; transfer your personal data, e.g. We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone. Sulfur has total four valance electrons and all these valance electrons are getting paired by the four valance electrons coming from four chlorine atoms. WebChemistry Chemistry questions and answers What atomic or hybrid orbitals make up the sigma bond between Si and Cl in silicon tetrachloride, SiCl4? Molecular weight: 169.898. [all data], Jain and Yadav, 1973, 2 Is SiCl4 Polar or Nonpolar? Purified tetrachlorosilane is used as a raw material for the production of optical fibres, silicon wafers and semiconductors. The information reflects Due to absence of this repulsion, the molecule shows its actual geometrical structure which can be predicted by using only the factor hybridization. They may also produce flammable gaseous H2. The personal data administrator is PCC Rokita SA with its registered office in Brzeg Dolny (Sienkiewicza Street 4, 56-120 Brzeg Dolny). The product is also used as a cross-linking agent in the processing of styrene-butadiene rubber (SBR). WebSilicon tetrachloride. Quality control of any optical fibre manufacturing process starts with the suppliers of the chemicals used as the raw materials for the substrate rods, chemical reactants and fibre coatings. We will calculate the formal charge on the 4th step structure to verify its stability. Dipole moment generated along with the bond(Si-Cl) due to the separation of charge induced on atoms, this charge is induced because the electronegativity of chlorine is 3.16 and for silicon, it is 1.90. According to the lewis structure of SiCl4, the central atom(silicon) doesnt contain any lone pair on it. Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. The structure with the formal charge close to zero or zero is the best and stable lewis structure. What changes are coming? National Ocean Service, on chlorine atom = (7 6 2/2) = 0. Silicon has zero electrons as nonbonded and each of the chlorine atom has six electrons as nonbonding. So, in the case of SiCl4, from silicon and chlorine, silicon(1.8) is less electronegative than chlorine(3.16), as electronegativity increases from left to right across a period in the periodic table. The above steps must be followed to draw a lewis structure. It is decomposed by water to hydrochloric acid with evolution of heat. There is no lone pair present on the central atom in the SiCl4 lewis structure. precursor in the semiconductor production proces. The number of electrons in each of Silicon's shells is 2, 8, 4 and its electron configuration is [Ne] 3s 2 3p 2. The difference of the electronegativity between these atoms high, this makes a Si-Cl polar covalent bond in nature. The Vapor Pressure of Silicon Tetrachloride, WebThe meaning of SILICON TETRACHLORIDE is a colorless fuming corrosive liquid SiCl4 made usually by heating silicon or silicon carbide with chlorine and used chiefly for We had 24 valence electrons left and in the above structure, we used (6 valence electrons 4 chlorine atoms) = 24 valence electrons. endstream Silicon tetrachloride is an inorganic compound that appears as a colorless liquid with a pungent odor having the chemical formula SiCl4. uuid:3a15fec3-7441-455d-98cc-018dff7ed151 Chloralkali, raw materials and intermediatesChlorosilanes, raw materials and intermediatesSpecialty Products / Specialty additives See answer Advertisement pstnonsonjoku It is miscible with toluene, benzene, chloroform, carbon tetrachloride, petroleum ether, ether and hydrochloric acid. Sauer, R.O. ["surowiec-dla-krzemionki-plomieniowej","funkcje-surowce-i-polprodukty-chemiczne"] : 10026-04-7. But the same methods are not effective if we want to get rid of hydrogen impurities. Therefore, we are left with 24 valence electrons more. errors or omissions in the Database. WebOther names:Silane, tetrachloro-;Silicon chloride (SiCl4);Tetrachlorosilane;Tetrachlorosilicon;SiCl4;Silicon chloride;Silicon(IV) This product is completely Maxsperse 8900/100M was developed as adispersing agents for use in most polyolefins. The electron geometry for SiCl4 is also tetrahedral. var e = document.getElementById("fdc9f57997a974af2a5836556275d729d"); This material is incompatible with alkali metals and dimethyl sulfoxide. Devyatykh, G.G. The overall formal charge in Silicon tetrachloride is zero. }