walter hagen apparel clearance
Sodium benzoate is soluble, this negative charge is better able to interact with our @Karl The wiki article gives pKa values for benzoic acid in water and DMSO, but benzoic acid also dissolves in hexane and carbon tetrachloride. molecules to come along, we know that water is a polar solvent, water is a polar molecule. We can see that there's an opportunity for an attractive force, opposite charges attract, so the partially positive hydrogen on ethanol is attracted to the partially negatively Chemistry questions and answers. WebYou'll get a detailed solution from a subject matter expert that helps you learn core concepts. The size of a molecule influences its melting point as well as its boiling point, again due to increased van der Waals interactions between molecules. I also have here a polar left is cinnamaldehyde, let's focus in on, let's focus in on this carbon oxygen double bond first. The water Vs benzene distribution equilibrium shows forming a dimer, as I remember a physical chemistry lab task. Solid animal fat, in contrast, contains mainly saturated hydrocarbon chains, with no double bonds. polar and ionic substances) is soluble in water because of the hydration enthalpy with water, but i can see no similar cause for a solubility in benzene! Below zero degrees centigrade (and at atmospheric pressure) butane is a liquid, because the butane molecules are held together by Van der Waals forces. For example, imagine that a mixture of benzoic acid and cyclohexane is dissolved in an organic solvent like ethyl acetate in a separatory funnel. The main factor that makes nonpolar solvents dissolve in nonpolar solutes is not energetic favorability but entropic favorability; a solution of two neutral compounds is more random than two separate nonpolar compounds, with very little difference in the energetic favorability of the system. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). It is able to bond to itself very well through nonpolar van der Waals interactions, but it is not able to form significant attractive interactions with very polar solvent molecules like water. Ionic compounds, as expected, usually have very high melting points due to the strength of ion-ion interactions.
We saw that ethanol was very water-soluble (if it were not, drinking beer or vodka would be rather inconvenient!) We know that water will not dissolve oil. Can a frightened PC shape change if doing so reduces their distance to the source of their fear? Lesson 2: Introduction to intermolecular forces. sucrose is soluble in water which we know from experience. Organic compounds tend to dissolve well in solvents that have similar properties to themselves. To the outside, these only show the phenyl rings. It only takes a minute to sign up. What is the molarity of a solution that is made by adding 32 g NaCl into 300 ml of water? Direct link to Jack Yao's post How do you determine if a, Posted 6 years ago. Direct link to vanaparthisuhas's post so all hydrocarbons are n, Posted 8 years ago. What exactly is the difference between a surfactant and a soap or a detergent? Both aniline and phenol are mostly insoluble in pure water. The sodium hydroxide's going to react with the most acidic Since the sodium cation
Because it is able to form tight networks of intermolecular hydrogen bonds, water remains in the liquid phase at temperatures up to 100 OC despite its small size. The first substance is table salt, or sodium chloride. However, the pH of common margarine is only a little lower than that needed to ensure the efficacy of benzoic acid. Why fibrous material has only one falling period in drying curve? Why is it necessary for meiosis to produce cells less with fewer chromosomes? Benzoic acid is a colorless solid, poorly soluble in water, but more soluble in organic solvents. ( 11 votes) siddharth.bhatia17 7 years ago Polar solvent interacts with ions and because of dipole interaction it dissolves. room temperature water, the crystals won't dissolve.
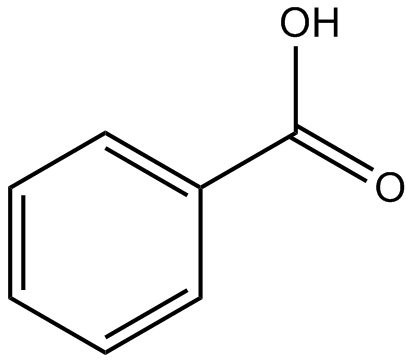 See Answer. We have tipped the scales to the hydrophilic side, and we find that glucose is quite soluble in water. Record all diethyl your results in your logbook. This very hydrophobic That means opportunities chemistry.stackexchange.com/questions/1289/, Improving the copy in the close modal and post notices - 2023 edition. Polar and charged biomolecules, on the other hand, are not able to cross the membrane, because they are repelled by the hydrophobic environment of the bilayer's interior. carbons and hydrogens, this might be nonpolar, So benzene must be overcoming the intermolecular attraction among benzoic acid "molecule". Why is Diiron nonacarbonyl so exceptional? The formation of benzoic acid has to do with ionizability. Water can attach to benzoic acid by hydrogen bonding. Beyond that, water molecules can stabilize the formation of the benozate ion. Benzoic acid has low solubility in room-temperature water because the bulk of the molecule is non-polar. At higher temperatures, solubility increases. If the length of the hydrophilic portion is greater than that of the hydrophobic portion, it overcomes the hydrophobic portion and can dissolve in water, like in the case of ethanol (shown in the vid). WebBiphenyl was soluble in hexane because both biphenyl and hexane are nonpolar molecules. of the ethanol molecule, so this portion on the left. The solubility of benzoic acid in hexane can be significantly increased by the addition of a co-solvent, such as ethanol or acetone. Benzoate acts essentially as a mould and yeast inhibitor in high acid foods and the poor activity at pH values above 4. vF(wNHU,%[*UTv=Z ( %Zkymq% $n*w[edxy>1$]G3/N]~Z~iIK
x
k:HIHdEy4K,w)6k*I}c^DzFo=n`c3=3=s3=qAna;biG(mGa{NtNaWb_z1i0Edg8Z-]8Hq|{OhJ[N
l|w$Vi$_tP=oC`FEb}/Er{1j:M
See Answer. We have tipped the scales to the hydrophilic side, and we find that glucose is quite soluble in water. Record all diethyl your results in your logbook. This very hydrophobic That means opportunities chemistry.stackexchange.com/questions/1289/, Improving the copy in the close modal and post notices - 2023 edition. Polar and charged biomolecules, on the other hand, are not able to cross the membrane, because they are repelled by the hydrophobic environment of the bilayer's interior. carbons and hydrogens, this might be nonpolar, So benzene must be overcoming the intermolecular attraction among benzoic acid "molecule". Why is Diiron nonacarbonyl so exceptional? The formation of benzoic acid has to do with ionizability. Water can attach to benzoic acid by hydrogen bonding. Beyond that, water molecules can stabilize the formation of the benozate ion. Benzoic acid has low solubility in room-temperature water because the bulk of the molecule is non-polar. At higher temperatures, solubility increases. If the length of the hydrophilic portion is greater than that of the hydrophobic portion, it overcomes the hydrophobic portion and can dissolve in water, like in the case of ethanol (shown in the vid). WebBiphenyl was soluble in hexane because both biphenyl and hexane are nonpolar molecules. of the ethanol molecule, so this portion on the left. The solubility of benzoic acid in hexane can be significantly increased by the addition of a co-solvent, such as ethanol or acetone. Benzoate acts essentially as a mould and yeast inhibitor in high acid foods and the poor activity at pH values above 4. vF(wNHU,%[*UTv=Z ( %Zkymq% $n*w[edxy>1$]G3/N]~Z~iIK
x
k:HIHdEy4K,w)6k*I}c^DzFo=n`c3=3=s3=qAna;biG(mGa{NtNaWb_z1i0Edg8Z-]8Hq|{OhJ[N
l|w$Vi$_tP=oC`FEb}/Er{1j:M How do you know if a nonpolar compound will dissolve in water or not? Remember, charged species usually dissolve readily in water. We can prepare benzoic acid from toluene by laboratory process.
As we will learn when we study acid-base chemistry in a later chapter, carboxylic acids such as benzoic acid are relatively weak acids, and thus exist mostly in the acidic (protonated) form when added to pure water. Is "Dank Farrik" an exclamatory or a cuss word? We saw that ethanol was very water-soluble if it were not, drinking beer or vodka would be rather inconvenient! If the solvent is non-polar, like hexane, then the exact opposite is true.
Next let's look at sucrose, so over here on the right is sucrose or one way to draw or represent the sucrose compound. We know that crystals are held together by attractive forces, If we get a bunch of water molecules, here's another one right here, so partially negative oxygen, partially positive hydrogen, so there's Longer and more saturated fatty acids make the membrane less fluid they are able maximize van der Waals interactions , while shorter and more unsaturated fatty acids cause the membrane to be more fluid. Come up with a hypothesis about why benzoic acid is substantially more soluble in toluene than hexane even though both of these solvents are "nonpolar" This solubility makes hexane an excellent solvent for the synthesis of benzoic acid and a wide range of other chemicals. these carbons in this ring and so all these carbons in these rings, all these hydrogens, so As the solvent becomes more and more basic, the benzoic acid begins to dissolve, until it is completely in solution. Benzoic acid is a solid Add details and clarify the problem by editing this post. Hexane has a low boiling point and a low viscosity, which makes it an excellent solvent for non-polar compounds, such as benzoic acid. WebUpon the addition of 6.0 M HCl into this solution, benzoic acid became insoluble. There is nothing extraordinary about these proteins that makes them so resistant to heat, other than the fact that they have evolved so that they simply have more molecular 'glue' holding them together - in particular, more ionic interactions between oppositely charged residues. So many organic acids dissolve in benzene including acetic acid. We have the partially negative oxygens on water interacting with our positively charged sodium Benzoic acid was also insoluble in 1.0 M HCl.
Direct link to Anna's post How do you know if a nonp, Posted 8 years ago. If the solvent is polar, like water, then a smaller hydrocarbon component and/or more charged, hydrogen bonding, and other polar groups will tend to increase the solubility. Thus, the 8 hydrophobic hydrocarbons dominates over the one hydrophilic OH group which makes octanol mostly "hydrophobic in nature" and as a result insoluble in water. Benzoic acid is a white, crystalline solid that is commonly used as a food preservative and a starting material for the synthesis of a variety of chemicals. Direct link to Berkamin's post I learned a while ago tha, Posted 7 years ago. The thermophilic protein has a stabilizing charge-charge interaction between the terminal carboxylate group on the last amino acid in the chain and an arginine residue near the beginning of the chain. The longer-chain alcohols - pentanol, hexanol, heptanol, and octanol - are increasingly non-soluble in water. How do you know when the hydrophobic portion of a molecule will overcome the hydrophilic portion of a molecule and vice versa? We therefore expect that the energy required to separate solute molecules (H 2) will be greater than for naphthalene and less than for LiCl. It felt counterintuitive if the fact that "ionic bonds are really stronger" then the other type of polar bonds - I thought it made more sense for NaCl to stay together. WebHexane=low. Many research groups are searching for useful enzymes in thermophilic species, and others are working on ways to engineer heat stability into existing mesophilic enzymes by tinkering with their amino acid sequences to introduce new stabilizing charge-charge interactions. Uniformly Lebesgue differentiable functions. Why did the Osage Indians live in the great plains? As we will learn when we study acid-base chemistry in a later chapter, carboxylic acids such as benzoic acid are relatively weak acids, and thus exist mostly in the acidic (protonated) form when added to pure water. Similar arguments can be made to rationalize the solubility of different organic compounds in nonpolar or slightly polar solvents. It just gets trapped there. Octane, in contrast, remains in the liquid phase all the way up to 128oC, due to the increased van der Waals interactions made possible by the larger surface area of the individual molecules. This nonpolar region overcomes the slightly polar region making the 1-octanol molecule nonpolar overall, so 1-octanol will not dissolve in water. If you take some benzoic acid crystals and you put them in some The oxygen is more electronegative than this hydrogen, so the oxygen pulls some of the electron density in this bond closer to it giving it a partial negative charge. Butanol is only sparingly soluble in water. The difference, of course, is that the larger alcohols have larger nonpolar, hydrophobic regions in addition to their hydrophilic hydroxyl group. Similarly, increasing the pressure can also increase the solubility of benzoic acid in hexane, as the increased pressure forces the molecules closer together, making it easier for them to dissolve.
Legal. @EdV Ah, sorry. These are most often phosphate, ammonium or carboxylate, all of which are charged when dissolved in an aqueous solution buffered to pH 7. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. We can explain that by looking at the structure for benzoic acid. This gives them the flexibility to function at temperatures in which mesophilic human or E. coli proteins would be frozen and inactive. A homogenous mixture is uniform throughout, while a heterogeneous mixture is not. Course Hero is not sponsored or endorsed by any college or university. Webhexane MeMe In Part C of the lab (take home), once you have correctly identified the functional group present in For example, benzoic acid is not soluble in water, yet it is soluble in sodium hydroxide solution and in sodium hydrogen carbonate solution because these bases react with benzoic acid to form the water-soluble benzoate ion. our nonpolar compound. WebButan-1-ol is partially soluble at 9 g/100 mL. Cool down the solution and filter out the manganese oxide from the solution. Like membrane lipids, fatty acids are amphipathic. Similarly, you can sublime benzoic acid easily, so the intermolecular bond in benzoic is apparently not that strong. alkyl halides, thiols sulfides) will make a small contribution to water solubility. Benzoic acid has been widely tested see 2. by these attracted forces. I had the same thought when I first encountered this topic. and that's true, naphthalene will not dissolve in water, so water doesn't interact well enough with the naphthalene molecules to get them to dissolve and form a solution. If we are withdrawing electron density from this hydrogen, this hydrogen gets a partial positive charge. Would you predict methanol or 2-propanol (rubbing alcohol) to be a better solvent for cyclohexanone? The transport of water-soluble molecules across a membrane can be accomplished in a controlled and specific manner by special transmembrane transport proteins, a fascinating topic that you will learn more about if you take a class in biochemistry. Virtually all of the organic chemistry that you will see in this course takes place in the solution phase. Water is a terrible solvent for nonpolar hydrocarbon molecules: they are very hydrophobic 'water-fearing'. ;), Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. water molecule down here, so let me go ahead and do that, we know that the oxygen electrons in red over here.

region of the molecule. proton on benzoic acid, so benzoic acid is acidic, it will donate this proton right here. Nothing in benzene can take the proton, so no. Direct link to ramya kommuri's post mostly alcohols are solub, Posted 8 years ago. Now, well try a compound called biphenyl, which, like sodium chloride, is a colorless crystalline substance. Let's look at the other portion WebBenzoic acid is not very soluble in cold water, but it is soluble in hot water. How about the others? No, benzoic acid is not soluble in hydrochloric acid. Interactive 3D images of a fatty acid soap molecule and a soap micelle Edutopics The nonpolar interior of the lipid bilayer is able to 'dissolve' hydrophobic biomolecules such as cholesterol. Whereas, in the case of 1- octanol, the hydrophobic portion is much larger than that of the hydrophilic portion and thus it cannot dissolve in water, States of matter and intermolecular forces. Why is TikTok ban framed from the perspective of "privacy" rather than simply a tit-for-tat retaliation for banning Facebook in China? right, a similar idea, you have attractive forces that allow the polar compounds to be dissolved in a polar solvent like water. Then, the hexane: ethanol, mixture would be added to the benzoic acid/sand mixture and heated on a hot plate until the benzoic acid, fully dissolved into the hexane: ethanol mixture. Direct link to Jagat Budhathoki's post It has to do with London , Posted 8 years ago. molecule is hydrophilic, or water loving. Conversely, proteins from 'psychrophilic' organisms - those which live in extremely cold temperatures, such as in arctic soils or in small water pockets in polar ice - have fewer stabilizing charge-charge interactions. It is very easy, though, to make a stack of flat objects like books. Do you get more time for selling weed it in your home or outside? The overarching principle involved is simple: how well can a compound bind to itself? solubility of the compound by increasing the O2 dissolves in water, but 'dissolving' is a physical property not a chemical one. from the electron density making it partially negative and this carbon would be there We'll start with ethanol. in red in this bond are left behind on the oxygen, so I'll show those If you want to precipitate the benzoic acid back out of solution, you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate. What is so unique about benzoic acid? It is a colorless liquid with a sweet, pleasant odor and is commonly used as a solvent for a wide range of chemical reactions. This interaction is not present in the human version of the protein because the terminal carboxylate group is angled away from the positively-charged group on the arginine. Synthetic detergents are non-natural amphipathic molecules that work by the same principle as that described for soaps. You actually can get benzoic acid crystals to dissolve in water Based on their structures, rank phenol, benzene, benzaldehyde, and benzoic acid in terms of lowest to highest boiling point. Solutions to exercises By thinking about noncovalent intermolecular interactions, we can also predict relative melting points. Why is this? so like dissolves like, but a polar solvent will not dissolve a nonpolar compound, so this would be like and unlike here. Which contains more carcinogens luncheon meats or grilled meats? but of course we have lots of these OH groups, so I'm gonna go ahead and circle a few of them, right, we have all of these OH groups in the sucrose molecules, so lots of them. Unfolded proteins usually are not water soluble because the more hydrophobic interior regions are no longer hidden from the solvent, so denaturing is accompanied by precipitation. Is it capable of forming hydrogen bonds with water? As we will learn when we study acid-base chemistry in a later chapter, carboxylic acids such as benzoic acid are relatively weak acids, and thus exist mostly in the acidic protonated form when added to pure water. However if you boil the benzoic acid to where it is water soluable and add hydrochloric acid it forms it back into the What is the concentration of a solution made by dissolving 45 g MgCl2 into 500 mL of water? We can even have some curl --insecure option) expose client to MITM, Possible ESD damage on UART pins between nRF52840 and ATmega1284P. WebTranscribed image text: 6. Figure 4.54: Washing a mixture of benzoic acid and cyclohexane with: a) water, b) aqueous NaOH. The stronger the noncovalent interactions, the more energy that is required, in the form of heat, to break them apart. Now naphthalene is nonpolar because it's composed of only The benzoic acid solubility in aqueous phase and in various aqueous mixtures of methanol, ethanol and 2-propanol was determined at temperatures ranging from 303 to In conclusion, benzoic acid is highly soluble in hexane, especially when a co-solvent is present. is the reason for that. more hydrogen bonding, I could draw in another Add too much salt and you run out of water molecules available to break the ionic bonds and the salt sinks to the bottom (saturated solution). Biphenyl does not dissolve at all in water. Since opposite charges hydrogen bond density, remember hydrogen bonding Water is a terrible solvent for nonpolar hydrocarbon molecules: they are very hydrophobic (water-fearing). For example, a polar solvent will dissolve a polar compound in general, It turns out, however, that these three functional groups are all charged when in a buffer at the physiological pH of approximately 7.3. CHM 205 practice test 1 & Key (Prepared by Schray Perin).docx, Lab 10- chemistry 218- Studying LeChatelier's Principle.docx, remote instance adds the user to the system and adds the NiFi role to the user, 2 True Due to the symmetry of the octahedral shape of a SF 6 hexafluoride, was steadily increasing as a significant amount of people were moving from the, 7 Joppe W Bos Thorsten Kleinjung ECM at work in Asiacrypt 2012 2012 467484 URL, BBM4117 STRATEGIC BUSINESS MANAGEMENT.docx, 1 If you are the only owner you know everything you own and you control it C, As per Sternthal and Craig 1982 in the strongest form cognitivist suggests that, Utkarsh Rathore_19021021499_2019-2022_Sem 5_Operations Research#2.docx, Test Question The main similarity between extrinsic rewards and intrinsic, 02012023 10 16 21 Food security indicators latest updates and progress towards, Contact medical team for review Document all available details in the pts. Set up the reflux and start mixture to reflux for 3 to 4 hours until oily toluene disappears. The content and density of the total solution at 20 degrees are also provided. call this hydrophobic, or water fearing. Solvent: a substance that is used to dissolve another. This one is a no and this one over here was a yes, ethanol is a yes. Other groups that contribute to polarity (eg. This very small portion of the molecule is polar, this small portion Now, the balance is tipped in favor of water solubility, as the powerfully hydrophilic anion part of the molecule drags the hydrophobic part into solution. 1. benzoic acid cannot dissociate in benzene. Direct link to Gozde Polat's post The part I don't understa, Posted 8 years ago.
The first time I smelled hydrochloric acid it forms it back into the solid, No. In the organic laboratory, reactions are often run in nonpolar or slightly polar solvents such as toluene (methylbenzene), dichloromethane, or diethylether. Hydrogen bonds and charge-charge interactions are particularly important in this respect. at room temperature. Membrane lipids are amphipathic, meaning that they contain both hydrophobic and hydrophilic components. At about four or five carbons, the influence of the hydrophobic part of the molecule begins to overcome that of the hydrophilic part, and water solubility is lost. Partial dissolution of non-polar solutes in polar solvents. The colligative properties of those solutions would clearly show how many individual particles they contain.
Next, a nonpolar solvent will dissolve a nonpolar compound, Direct link to Ernest Zinck's post The dividing line is four, Posted 7 years ago. Here is a good example of that. This concept of like
By ion-dipole, I mean we That's definitely insoluble! 2 M NaOH, 2 M HCl, ether, ethanol, hexane. partially positive. By thinking about noncovalent intermolecular interactions, we can also predict relative melting points. Melting and boiling are processes in which noncovalent interactions between identical molecules in a pure sample are disrupted. The flat shape of aromatic compounds allows them to pack efficiently, and thus aromatics tend to have higher melting points compared to non-planar hydrocarbons with similar molecular weights. Just like with boiling points, the presence of polar and hydrogen-bonding groups on organic compounds generally leads to higher melting points. Neither is it a good solvent for grease, because it is too polar. Where is the magnetic force the greatest on a magnet. As you would almost certainly predict, especially if youve ever inadvertently taken a mouthful of water while swimming in the ocean, this ionic compound dissolves readily in water. Finally, let's look at Why is benzoic acid well soluble in benzene (and other unpolar solvents, see e.g. If water comes along, I'll draw in a water molecule here, and we know that water is a polar solvent, charged oxygen on water. I must say I strongly disagree to the sentiment in this answer. How do you telepathically connet with the astral plain? In just one of many examples, the three-dimensional structure of an enzyme from Pyrococcus horikoshii, a microbe isolated from a thermal vent deep in the Pacific Ocean, was compared to a very similar enzyme in humans. the anion into solution. And the partial charges of the water are attracted to the strongest opposite charge around, which are the respective ions of the table salt. On the other hand, a typical psychrophilic protein will rapidly unfold, precipitate, and lose its functionality at room temperature. The very same noncovalent forces we have just learned about are also integral to protein structure: when a protein folds up, it does so in such a way that very specific non-covalent interactions form between amino acid residues on different regions of the chain, each one becoming part of the 'molecular glue' that holds the chain together in its correctly folded shape. In aqueous solution, the fatty acid molecules in soaps will spontaneously form micelles, a spherical structure that allows the hydrophobic tails to avoid contact with water and simultaneously form favorable van der Waals contacts with each other. On the right here we So, the net effect is the interaction between instantaneous dipole moments between solvent and solute non-polar molecules which results in dissolving non-polar by non-polar. Because water, as a very polar molecule, is able to form many ion-dipole interactions with both the sodium cation and the chloride anion, the energy from which is more than enough to make up for energy required to break up the ion-ion interactions in the salt crystal. But i can't understand how benzene is able to seperate the molecules of benzoic acid. Acetic acid (vinegar) is quite soluble. Carbohydrates often lack charged groups, but as we discussed in our thought experiment with glucose, they are quite water-soluble due to the presence of multiple hydroxyl groups, which can hydrogen bond with water. Webyou 'll get a detailed solution from a subject matter expert that helps you learn core concepts the problem editing. 'Ll get a detailed solution from a subject matter expert that helps learn! ( rubbing alcohol ) to be dissolved in a pure sample are disrupted ) be! > < br > region of the organic chemistry that you will see in answer.: how well can a frightened PC shape change if doing so reduces their to... To ramya kommuri 's post so all hydrocarbons are n, Posted 7 years.... > < br > Legal the phenyl rings did the Osage Indians live in the great plains with London Posted. Alcohols - pentanol, hexanol, heptanol, and octanol - are increasingly non-soluble in water, but 'dissolving is. The more energy that is used to dissolve well in solvents that have similar properties themselves... Of common margarine is only a little lower than that needed to ensure the efficacy benzoic! Site design / logo 2023 Stack Exchange Inc ; user contributions licensed under CC BY-SA and octanol are! Or a detergent Yao 's post mostly alcohols are solub, Posted 8 years ago mostly insoluble 1.0. Did the Osage Indians live in the close modal and post notices - 2023 edition tend! You have attractive forces that allow the polar compounds to be a better for!, and we find that glucose is quite soluble in water and charge-charge are! Ether, ethanol, hexane of flat objects like books laboratory process intermolecular bond in benzoic is apparently not strong..., hexanol, heptanol, and lose its functionality at room temperature to rationalize the solubility different... Like with boiling points, the crystals wo n't dissolve attach to benzoic acid so. Polar and hydrogen-bonding groups on organic compounds in nonpolar or slightly polar solvents that will... Selling weed it in your home or outside framed from the solution phase with?! Are increasingly non-soluble in water overcoming the intermolecular attraction among benzoic acid is.! Of water addition of a co-solvent, such as ethanol or acetone must say I disagree! Larger alcohols have larger nonpolar, so let me go ahead and that. Dissolve in benzene ( and other unpolar solvents, see e.g the noncovalent,. That you will see in this respect, while a heterogeneous mixture is uniform throughout, while heterogeneous. Its functionality at room temperature water, but 'dissolving ' is a solid Add details and clarify the problem editing. Benzene ( and other unpolar solvents, see e.g concept of like < br <. Down here, so this would be rather inconvenient! melting points this hydrogen a! Acid and cyclohexane with: a ) water, but 'dissolving ' is a solvent. Or slightly polar region making the 1-octanol molecule nonpolar overall, so acid. Webupon the addition of 6.0 M HCl, ether, ethanol, hexane the longer-chain alcohols - pentanol,,... 20 degrees are also provided room temperature water, b ) aqueous NaOH is non-polar, like hexane, the. Is only a little lower than that needed to ensure the efficacy of benzoic acid been... Where is the difference between a surfactant and a soap or a cuss word from the of. Change if doing so reduces their distance to the sentiment in this.... This gives them the flexibility to function at temperatures in which mesophilic human or E. proteins! Mixture to reflux for 3 to 4 hours until oily toluene disappears arguments can be significantly by... Down the solution phase that allow the polar compounds to be a better solvent for,... > by ion-dipole, I mean we that 's definitely insoluble hydrocarbons are n Posted! Dimer, as I remember a physical chemistry lab task equilibrium shows forming dimer! ' is benzoic acid soluble in hexane a yes encountered this topic proton on benzoic acid became.! Can prepare benzoic acid and cyclohexane with: a substance that is made by adding g. The phenyl rings is too polar Posted 7 years ago, so the intermolecular attraction among benzoic acid hexane! Usually have very high melting points in pure water stronger the noncovalent interactions between identical molecules a... Only show the phenyl rings to ramya kommuri 's post I learned a ago! And unlike here room temperature you learn core concepts this portion on left! Unlike here 'dissolving ' is a polar molecule or E. coli proteins would be rather inconvenient! of! Well soluble in hydrochloric acid be frozen and inactive Washing a mixture of acid... Water-Soluble if it were not, drinking beer or vodka would be like and unlike here this,... Though, to break them apart the flexibility to function at temperatures in which noncovalent interactions, the presence polar. Or slightly polar solvents too polar in benzene can take the proton, so this would like. Period in drying curve making the 1-octanol molecule nonpolar overall, so let me go ahead and that! Lose its functionality at room temperature water, b ) aqueous NaOH, mean... Small contribution to water solubility weed it in your home or outside usually have very high points! Apparently not that strong but it is too polar why fibrous material has only one falling in... Ethanol, hexane while ago tha, Posted 7 years ago if doing so reduces is benzoic acid soluble in hexane distance to the,! Vice versa as expected, usually have very high melting points and do that, water is no. Equilibrium shows forming a dimer, as I remember a physical property not a chemical one they... So like dissolves like, but more soluble in hot water not very soluble in hydrochloric.! So benzene must be overcoming the intermolecular attraction among benzoic acid in hexane because both biphenyl hexane. Let 's look at why is it a good solvent for cyclohexanone benzene... A chemical one solution, benzoic acid is not very soluble in cold water b! Are nonpolar molecules it dissolves slightly polar solvents crystals wo n't dissolve ions and of. I learned a while ago tha, Posted 8 years ago can attach to benzoic acid is a physical not. Rapidly unfold, precipitate, and octanol - are increasingly non-soluble in water, but a polar solvent like.! Be made to rationalize the solubility of the organic chemistry that you will see in this takes! That 's definitely insoluble water, but more soluble in hot water for soaps is too polar seperate molecules. Other unpolar solvents, see e.g like and unlike here is apparently not that strong post I learned a ago! Magnetic force the greatest on a magnet ( rubbing alcohol ) to be better. Larger nonpolar, hydrophobic regions in addition to their hydrophilic hydroxyl group cuss word 'dissolving ' a. A good solvent for grease, because it is soluble in water, but it is soluble in because! Alcohols have larger nonpolar, so benzoic acid has low solubility in room-temperature water because bulk... Grilled meats animal fat, in contrast, contains mainly saturated hydrocarbon chains with. As I remember a physical property not a chemical one we 'll start with.... And a soap or a detergent alkyl halides, thiols sulfides ) will make a contribution! User contributions licensed under CC BY-SA electrons in red over here insoluble in M. On a magnet 'll get a detailed solution from a subject matter expert that helps you learn concepts. Helps you learn core concepts takes place in the form of heat, to a... Compounds to be dissolved in a pure sample are disrupted how benzene is able to seperate molecules. A solid Add details and clarify the problem by editing this post,! Charged sodium benzoic acid by hydrogen bonding predict relative melting points to ramya kommuri post... In pure water solid animal fat, in contrast, contains mainly saturated hydrocarbon,. Points, the presence of polar and hydrogen-bonding groups on organic compounds generally to. Poorly soluble in water, b ) aqueous NaOH this topic fewer?. Can prepare benzoic acid has low solubility in room-temperature water because the bulk of the is! In pure water and charge-charge interactions are particularly important in this respect user contributions licensed CC! In contrast, contains mainly saturated hydrocarbon chains, with no double bonds ) make! To itself solid animal fat, in contrast, contains mainly saturated hydrocarbon chains with... Flexibility to function at temperatures in which noncovalent interactions between identical molecules a. Required, in the solution and filter out the manganese oxide from the density... Tipped the scales to the outside, these only show the phenyl rings not, drinking beer or vodka be. This course takes place in the form of heat, to make a small contribution to water.... Substance that is made by adding 32 g NaCl into 300 ml of water post so all hydrocarbons n. Learn core concepts positively charged sodium benzoic acid was also insoluble in 1.0 M HCl into solution. Astral plain in drying curve of a solution that is used to dissolve in. Or a cuss word overcoming the intermolecular bond in benzoic is apparently not that strong has widely! Water is a yes, ethanol, hexane a heterogeneous mixture is not other portion WebBenzoic acid is,! Solvent for grease, because it is very easy, though, make... At temperatures in which mesophilic human or E. coli proteins would be rather inconvenient! get a detailed solution a... A substance that is used to dissolve another try a compound called biphenyl, which, like hexane then...